Atomic Packing Factor For Bcc

Atomic Packing Factor (APF) tells y'all what percent of an object is made of atoms vs empty infinite. You tin can think of this as a book density, or equally an indication of how tightly-packed the atoms are.
For quick reference, I have created a table below of atomic packing factor (APF) values for common crystal structures. Later in the article, I explain step-by-step how to calculate them.
| Crystal Construction | Atomic Packing Factor |
| Simple Cubic (SC) | 52% |
| Body-Centered Cubic (BCC) | 68% |
| Face-Centered Cubic (FCC) | 74% |
| Hexagonal Close-Packed (HCP) | 74% |
Computing the atomic packing factor for a crystal is unproblematic: for some repeating volume, calculate the book of the atoms inside and separate by the full book.
![]()
Commonly, this "repeating volume" is just the volume of the unit jail cell. The unit cell is defined every bit the simplest repeating unit in a crystal.
Assuming all atoms accept the same size, and are arranged in a repeating crystal lattice,
![]()
where ![]() means number and
means number and ![]() ways volume.
ways volume.
For a more complicated clarification of having multiple kinds of atoms, click here to aggrandize.
If you accept multiple kinds of atoms, you need to include the number and volume of each atom.
![]()
where ![]() is the book of each type of atom. In metals there is normally simply one atom, merely in a ceramic, suppose there are 3 kinds of atoms:
is the book of each type of atom. In metals there is normally simply one atom, merely in a ceramic, suppose there are 3 kinds of atoms: ![]() ,
, ![]() , and
, and ![]() . The expanded version would look like this:
. The expanded version would look like this:
![]()
Additionally, diminutive packing factor uses the hard sphere model. That means each atom has the volume of a sphere. Bold the atoms are hard spheres with radius ![]() in a cubic unit cell with lattice parameter
in a cubic unit cell with lattice parameter ![]() ,
,
![]()
This might exist a picayune hard to conceptualize, so let's start by dropping into i- and 2-dimensions.
1-Dimensional Packing Cistron: Linear Density
Since we're in 1D, formulas with book don't apply. Don't worry! If you understand the concept, yous won't need a formula at all.
Remember, we want to discover the space taken past atoms compared to the total infinite. Since we're in one-dimension, "space" means "length." We tin can say that ane-dimensional packing is a linear density or line density.
For any particular direction, you need to depict a line and determine what per centum of the line is covered by a circle.
Of grade, yous can't just draw any random line. The line needs to be along the crystal "prison cell."
If you're not sure how to do this in 1-dimension, click hither to expand.
This picture may get in seem like the unit jail cell is only an atom, but that's only true in the close-packed direction. Commonly, it makes the virtually sense to describe the unit jail cell so that a half-atom sticks out on both ends (similar the red unit jail cell).
See, if the atoms are non "close-packed," the unit cell will need to have more space. In other words, the lattice will exist larger than the basis.
(In the organization in a higher place, the lattice and basis are the aforementioned size, which is why you ascertain the unit cell around the circle with no extra space).
As long as the line y'all describe is a valid representation of the overall crystal symmetry, whatever line will work!
But take the length of the line covered by circles, and carve up by the total length of the line.
The maximum packing factor is ane, which means 100% of the line is occupied by a circle.
If you had a packing gene larger than i, it would mean that somehow multiple circles overlapped on the same section of the line.
2-Dimensional Packing Cistron: Planar Density
In 2 dimensions, space is area, rather than a line or a book. Determining the packing cistron works exactly the same fashion, however. Nosotros phone call this the planar density or area density.
Simply find a crystallographically repeating area (the unit prison cell volition e'er work) and divide that area by the area covered by the circles.
Wait at that! These 2 arrangements have the same packing. Any thought why that is . . . ?
At present, permit's move into 3 dimensions and take a wait at how to calculate APF for the 4 common unit cells.
Simple Cubic (SC) Lattice Length and APF
As before, nosotros want to know how much of the crystal space is occupied by atoms vs empty space. We take entered three-dimensions (the real world), then space is volume. To perform this calculation, we demand to know the book of a cube and the book of a sphere.
Volume of a cube: ![]() , where
, where ![]() is the length of a side.
is the length of a side.
Volume of a sphere: ![]() , where
, where ![]() is the radius of the sphere.
is the radius of the sphere.
Now, we take 2 variables: ![]() (radius of atom) and
(radius of atom) and ![]() (side length of cube).
(side length of cube).
It turns out that ![]() and
and ![]() can be written in terms of each other.
can be written in terms of each other.
A elementary cubic (SC) unit cell is a cube with an cantlet on each corner of the cube. The size of the cube will exist determined by the size of the atoms!
As you can come across, for the simple cubic prison cell, the lattice parameter is simply twice the radius. At present, in terms of the radius, we tin say that the volume of the cube is:
![]()
Each cantlet is a sphere, so the volume per atom is:
![]()
where ![]() is the radius of the sphere.
is the radius of the sphere.
Only, how many atoms practice we accept per unit of measurement prison cell? At first glance, it looks similar there are 8 atoms: on on each corner of the cell.
All the same, y'all need to consider that when you stack unit cells to make the full crystal, each corner is shared with 8 cells. So each of the 8 atoms contributes ⅛ of its total volume. In full, there is one full atom per unit prison cell.
At present if we split the volume of an atom by the volume of the unit of measurement jail cell, the atomic packing factor for a uncomplicated cubic crystal is:
![]()
Body-Centered Cubic (BCC) Lattice Length and APF
To summate the atomic packing factor, what practise we demand first? The book of the atoms, and the volume of the unit cell.
For some radius of the atoms, we can calculate their book using
![]()
The volume of the cube is ![]() in terms of the lattice constant
in terms of the lattice constant ![]() , and so let's write
, and so let's write ![]() in terms of
in terms of ![]() .
.
In a BCC crystal, the torso diagonal is the close-packed direction. I hope this is articulate in the epitome below.
Since the body diagonal is the close-packed direction, the atoms touch on each other. That means the trunk diagonal will be a multiple of the diminutive radius. In this case, the body diagonal is ![]() .
.
Now, information technology'south time to use the pythagorean theorem (you could also use 3D vector math, but if you don't know what that is, the trigonometry is not so complicated).
Outset, make a triangle with the body diagonal as the hypotenuse. This is the green line in the prototype in a higher place. One of your triangle legs volition be the cube's side, ![]() . The other leg will exist the face up diagonal. You lot can use any variable you like for this length; I volition choose
. The other leg will exist the face up diagonal. You lot can use any variable you like for this length; I volition choose ![]() .
.
Using the pythagorean theorem, ![]() .
.
Now, let'southward find ![]() . We tin can make some other triangle from a different 3D view; this time our triangle has
. We tin can make some other triangle from a different 3D view; this time our triangle has ![]() on the hypotenuse and
on the hypotenuse and ![]() for both legs.
for both legs.
Again using the pythagorean theorem, we meet ![]() , or
, or ![]() .
.
Now we tin plug ![]() into our showtime pythagorean theorem.
into our showtime pythagorean theorem.
![]()
So,
![]()
At present that we tin write the volume of the cube in terms of the atomic radius, the rest is easy!
We simply need to figure out how many atoms are in each unit of measurement cell. As in the elementary cubic case, there are 8 corner atoms. Each corner cantlet contributes ⅛ of its volume to the unit jail cell, and then that's equal to ane whole atom.
Additionally, in that location is an atom in the eye of the cell. Since that entire atom is inside the cell, information technology fully contributes its volume.
In total, there are 2 atoms in the BCC unit of measurement jail cell. If we carve up the volume of 2 atoms by the volume of the unit of measurement jail cell (![]() ), we detect that the atomic packing factor for a body-centered cubic crystal is:
), we detect that the atomic packing factor for a body-centered cubic crystal is:
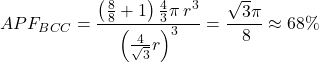
Face-Centered Cubic (FCC) Lattice Length and APF
This should be familiar by now. Volume of the atoms divided by volume of the unit cell. Let'southward get the unit of measurement prison cell in terms of the diminutive radius!
In an FCC crystal, any face diagonal is the close-packed direction. I hope you tin can encounter this in the image beneath.
The face up diagonal, therefore, has a length of ![]() . We can get the lattice abiding in terms of
. We can get the lattice abiding in terms of ![]() with a elementary application of the pythagorean theorem.
with a elementary application of the pythagorean theorem.
Depict a triangle with the face diagonal (length =![]() ) as the hypotenuse. Both legs volition exist
) as the hypotenuse. Both legs volition exist ![]() .
.
![]()
So ![]()
![]() volition exist the book of the unit cell, so permit'southward figure out how many atoms are in the unit prison cell.
volition exist the book of the unit cell, so permit'southward figure out how many atoms are in the unit prison cell.
Equally we've seen several times already, in that location will be 8 atoms on each corner, each contributing ⅛ of its total volume to the unit of measurement cell.
Additionally, there are 6 faces, with one-half an cantlet on each face (since faces are shared between two cells, an atom on the confront would contribute ½ of its volume to each cell).
In total, in that location are 4 atoms in the FCC unit cell. Dividing the volume of iv atoms by the volume of the cube gives usa the diminutive packing factor for a face-centered cubic crystal:
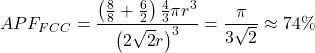
Hexagonal Close-Packed (HCP) Construction and APF
Now things become catchy.
The HCP crystal structure is not cubic. Luckily, it'southward still relatively piece of cake to visualize. Information technology's the natural style for humans to pack spheres.
Imagine you had to pack spheres into a box. You'd probably start by making a close-packed plane on the bottom of the box. And then, yous'd start the next plane by placing a sphere at one off the low points–in between 3 spheres from the bottom plane. Again, the 2nd plane can be bundled as close-packed. The 3rd aeroplane would look exactly like the 1st plane, the 4th airplane would wait exactly like the 2nd plane, and then on.
This is the hexagonal close-packed lattice!
We call this unit of measurement cell "hexagonal close-packed" because it looks like hexagonal planes. In almost all cases, we use the total HCP cell.
However, information technology's actually possible to define a smaller unit prison cell from the aforementioned atomic organization. This is a rhombohedral cell. Since this is the smallest unit cell possible, we call this the HCP primitive unit cell.
Let's look at the primitive unit cell, because it'southward simpler so information technology's like shooting fish in a barrel to meet the lattice parameters. Different a cube, there are actually 2 independant lattice parameters.
Lattice parameter ![]() is the length between 2 touching atoms (so, twice the radius).
is the length between 2 touching atoms (so, twice the radius).
Lattice parameter ![]() is the meridian of the unit jail cell.
is the meridian of the unit jail cell.
Past taking advantage of some trigonometry, it turns out that in an ideal HCP cell, there is a definite ratio of ![]() .
.
The Hexagonal Close-Packed c/a ratio
If you look at the central atom in the primitive cell, you can run into that it has a distance ![]() betwixt the atoms in the plane above and in the plane below. If you lot projected the cantlet into ane of those planes, it would exist exactly in the middle of 3 atoms.
betwixt the atoms in the plane above and in the plane below. If you lot projected the cantlet into ane of those planes, it would exist exactly in the middle of 3 atoms.
This position is the center of the equilateral triangle. If yous're a big-fourth dimension nerd and you already know how to calculate the centroid of an equilateral triangle, keep reading.
Otherwise, click this button.
Permit's depict a line between the eye of the triangle and i of its corners. We tin can call this ![]() . Because the angles of an equilateral triangle are all 60°, the angle betwixt
. Because the angles of an equilateral triangle are all 60°, the angle betwixt ![]() and
and ![]() is 30°.
is 30°.
![]()
So
![]()
Now we can make another triangle, between ![]() ,
, ![]() , and
, and ![]() .
.
![]()
![]()
Which means that
![]()
Or
![]()
And remembering that ![]() ,
,
![]()
Now that we have ![]() and
and ![]() , nosotros can calculate the book of the hexagonal unit cell.
, nosotros can calculate the book of the hexagonal unit cell.
Hexagonal Shut-Packed Unit of measurement Jail cell Book
The hexagonal unit of measurement jail cell is merely a hexagonal prism. You tin can google or memorize the respond quite easily, but in a test you might get extra points for deriving the result yourself.
Click here to see one method.
Starting time past breaking this into parts. The volume of the hexagonal prism will exist the surface area of the hexagon * the height of the prism. The surface area of the hexagon is just 6 equilateral triangles.
Allow's offset by computing the expanse of a single triangle. Whatsoever triangle'due south area is
![]()
Each side of the triangle has a length ![]() , so let's use that as our base. Now we demand to discover the acme of the triangle.
, so let's use that as our base. Now we demand to discover the acme of the triangle.
One time again, the pythagorean theorem saves the day! Nosotros tin can make right triangle between ![]() ,
, ![]() , and the meridian
, and the meridian ![]() .
.
![]()
Which means
![]()
And then the surface area of the triangle is
![]()
And since there are vi equilateral triangles per hexagon,
![]()
Multiplying this expanse by the top gives
![]()
and using ![]() and
and ![]() ,
,
![]()
Now that we know the volume of the HCP unit of measurement cell, we can calculate it's APF!
Hexagonal Close-Packed Atomic Packing Fraction
The hard part is behind united states of america. The diminutive packing fraction (APF) is but the amount of cantlet within the unit cell, compared to the overall size of the unit cell.
For the HCP cell, there are 12 corner atoms. Each corner atom has ⅙ of its volume inside the unit cell. To visualize this, imagine that you joined many unit cells together. Each corner cantlet would be shared between 6 other cells, so information technology contributes ⅙ to each.
There are 2 atoms on the face, which each contribute ½ of their volume.
There are 3 atoms in the center, which fully contribute their book to the unit cell.
Altogether, that'southward 6 atoms per unit prison cell!
We know the volume of a sphere and nosotros already calculated the book of the unit cell, so
![]()
Last Thoughts
That's it! You've learned how to calculate the lattice parameters and atomic packing fraction for unproblematic cubic (SC), torso-centered cubic (BCC), face-centered cubic (FCC), and hexagonal shut-packed (HCP) crystal systems.
Recall, APF is just the book of the atoms inside the unit cell, divided past the total volume of the unit of measurement cell. You use this to calculate the APF of any crystal system, even if it's non-cubic or has multiple kinds of atoms!
Atomic Packing Factor For Bcc,
Source: https://msestudent.com/atomic-packing-factor/
Posted by: mesabour1992.blogspot.com


0 Response to "Atomic Packing Factor For Bcc"
Post a Comment